FRACTIONAL DISTILLATION
FRACTIONAL DISTILLATION
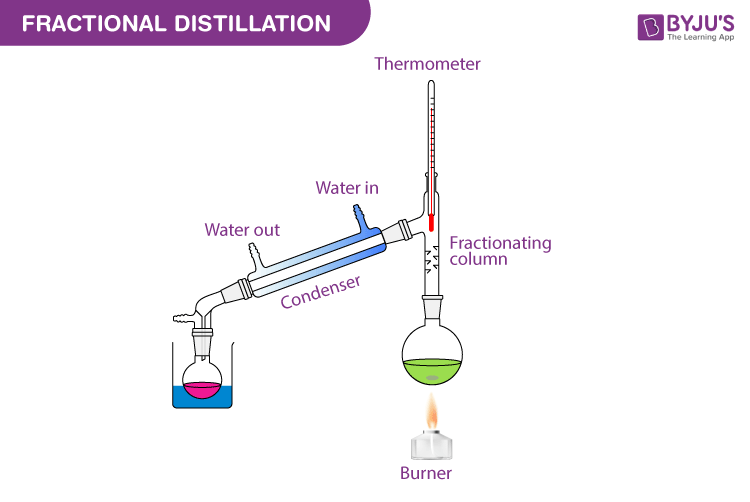
- Fractional distillation is used to separate a mixture of two or more liquid’s which are miscible and have boiling points that are close together (or separated by less than 40 C. Miscible liquids are liquids that can completely dissolve in each other forming a homogenous mixture.
- In fractional distillation a fractionating column is attached between your round bottom flask and the ‘Liebig condenser’. Fractional distillation can be used to separate is solution of ethanol and water since the boiling point of ethanol is 78 C and that of water is 100 C.
HOW FRACTIONAL DISTILLATION WORKS
- The mixture boils and vapors of both liquid enter and move up the fractionating column where they condensed and vaporized many times. As a mixture of vapors move up the fractionating column it becomes increasingly richer in the more volatile component i.e. the one of the lower boiling point (ethanol), until the vapour reaching the top of the column has the most volatile component. This vapour passes into the condenser, condenses and is collected as distillate.
- The vapor of the less volatile component, i.e. the compound with the higher boiling point ( water) condenses in the fractionating column and returns to the round bottom flask .
- Throughout the process, vaporization and condensation take place repeatedly until the two mixtures are separated completely.
USES OF FRACTIONAL DISTILLATION
Fractional Distillation is used for both oil refining and purification of reagents and products. Fractional distillation is used in oil refineries to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties. Gasoline, diesel fuel, kerosene, and jet fuel are some of the different fractions produced by an oil refinery.
