SIMPLE DISTILLATION
SIMPLE DISTILLATION
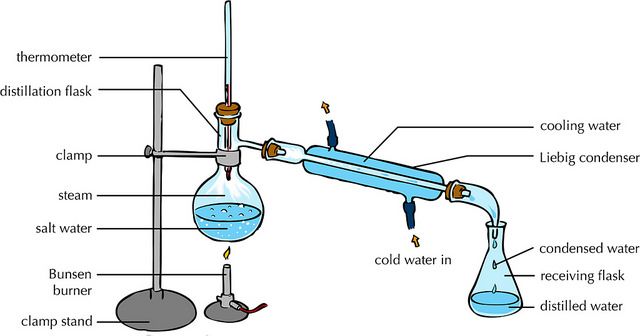
- The process of heating a liquid mixture to form vapor and then cooling that vapor to get a liquid.
- Simple distillation is used to separate a solution of a solid dissolved in a liquid. It allows for both the solid and liquid to be collected. Simple distillation is an appropriate method of separation only if the liquid has a lower boiling point than the solid, i.e. the liquid becomes a vapor before the solid.
- Simple distillation can be used to separate two liquids with different boiling points.
- An important part of the apparatus used for simple distillation it’s called a ‘Liebig condenser’.
SIMPLE DISTILLATION: METHOD
- The mixture in the distillation flask is brought to a boil and evaporation occurs.
- The liquid with the lowest boiling point will turn into a vapour passing into the Liebig Condenser where it will cool and condenses back to a liquid.
- The liquid passes down the condenser and is collected as the distillate.
- The concentration of the solution in the distillation flask gradually increases and when most of the liquid has vaporized, the solution can be poured into an evaporating basin and left to crystallize.
- A thermometer is used to monitor the temperature of the vapor entering the condenser. If the temperature remains constant at the boiling point of the pure liquid, then the distillate is pure.
USES OF DISTILLATION
- Water Purification: distilled water from tap water and, pure water from seawater (desalination).
- Production of some alcoholic beverages.
- Purification of reagents and products.
