PAPER CHROMATOGRAPHY
PAPER CHROMATOGRAPHY
Paper chromatography is used to separate a mixture of dissolved substances which are colored or can be coloured, and which will travel through a material, example filter paper.
The substances are separated based on
- How soluble they are in the solvent used
- How strongly they are attracted to the paper.
Many food colorings are mixtures of two or more dyes which can be separated by paper chromatography.
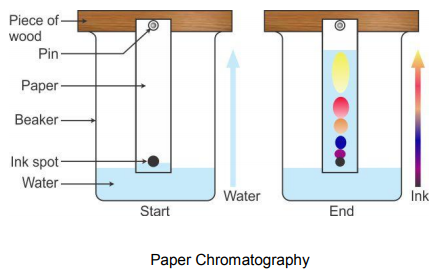
HOW DOES IT WORK?
A drop of mixture solution is spotted near one end of the paper and then dried. The end of the paper, nearest the spot, is then dipped into the solvent without submerging the spot itself.
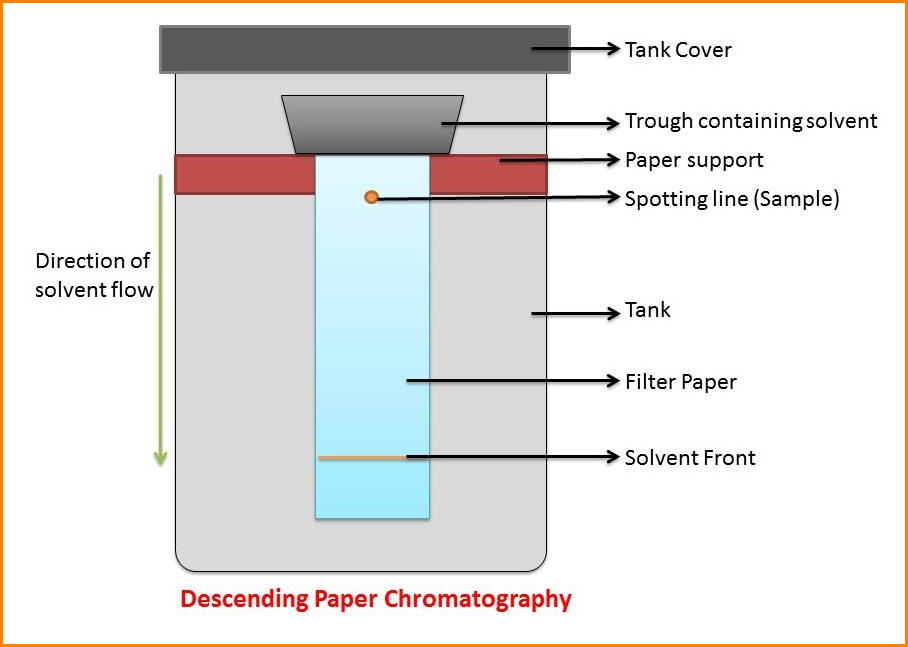
In ascending chromatography, the solvent is in a pool at the bottom and moves up by capillarity. In descending chromatography it is in a trough at the top and flows down by capillarity and gravity.
The solvent flows along the paper through the spots and on, carrying the substances from the spot. The solvent moves up the paper and dissolves the dyes in the mixture carrying them with it. However the dyes travel up the paper at different rates. The dyes that are most soluble in the solvent and less attracted to the paper travels the fastest and furthest distance.
The dyes that are the least soluble in the solvent and most attracted to the paper travels the slowest and the least distance. Once the solvent has completed its movement up the paper, the paper is allowed to dry. There will be a pattern of different colored dyes on the paper each one representing a part of the mixture. This pattern is known as a chromatogram.
The paper is called the stationary phase while the solvent is referred to as the mobile phase.
To get a measure of the extent of movement of a component in a paper chromatography experiment, we can calculate an “Rf value” for each separated component in the developed chromatogram.
An Rf value is a number that is defined as the distance traveled by the component from an application point.
